Thrips hawaiiensis (Morgan, 1913)
Thripinae, Thripidae, Terebrantia, Thysanoptera
Figures
Fig. 1: 8-segmented antenna, segments III and IV with forked sense cone, terminal segments V-VIII
Fig. 2: Head dorsal with ocellar triangle
Fig. 3: Pronotum
Fig. 4: Meso- and metanotum
Fig. 5: Fore wing and fore wing middle region
Fig. 6: Sternites VI and VII
Fig. 7: Tergites I-III
Fig. 8: Tergite VIII
Fig. 9: Tergites VIII-X (male)
Introduction and recognition
Thrips hawaiiensis a strict flower-dwelling species which can attack various crops such as banana, grapes, rose, tobacco. Both sexes fully winged. Body color brown or, in some populations, bicolored with head and thorax paler than brown abdomen; legs yellowish; antennal segment III yellow; fore wings brown with base paler. Antennae 7- or 8-segmented; segments III & IV slightly constricted at apex and with short forked sense cone, segments VII & VIII short (Fig. 1). Head wider than long; with 2 pairs of ocellar setae, pair I absent, pair III stout and arising outside of ocellar triangle; postocular setae pairs I & III slightly shorter than ocellar setae pair III, pair II about half as long as pair I (Fig. 2). Pronotum with 2 pairs of long posteroangular setae; posterior margin with 3-4 pairs of setae; anterior margin with 5-6 pairs of setae (Fig. 3). Mesonotum with lines of sculpture between and around campaniform sensilla near anterior margin; mesofurca with spinula. Metanotum with lines of sculpture transverse at anterior, and longitudinal and parallel or with equiangular reticulations on posterior half; median setae longer than lateral setae and arising at anterior margin; campaniform sensilla present (Fig. 4). Mid and hind tarsi 2-segmented. Fore wing first vein with 3 setae on distal half; second vein with about 14 closely set setae; clavus with 5 marginal setae and 1 discal seta, clavus subterminal seta shorter than the terminal seta (Fig. 5). Tergite II with 4 lateral marginal setae (Fig. 7); tergites V-VIII with ctenidia present laterally, on VIII posteromedial to spiracles; posterior margin of VIII with comb complete medially but microtrichia small and irregular and sometimes arising in groups (Fig. 8); pleurotergites without discal setae. Sternite II with 2 pairs of marginal setae, III-VII with 3 pairs, the median pair on VII arising in front of posterior margin; sternite II with 1-4 discal setae, III-VII with discal setae varying in number from 10-25 in a regular transverse row (Fig. 6).
Male similar to female in structure, but smaller and paler; tergite VIII with posteromarginal microtrichia medially or no marginal comb (Fig. 9); tergite IX with median S1 setae slightly longer than S2 and equidistant from S2 and from each other; sternites III-VII with transverse glandular area anterior to discal setae.
Taxonomic identity
Species
Thrips hawaiiensis (Morgan, 1913)
Taxonomic history
Taeniothrips florinatus Priesner, 1938
Taeniothrips rhodomyrti Priesner, 1938
Thrips imitator Priesner, 1934
Physothrips darci Girault, 1930
Physothrips lacteicolor Girault, 1928
Physothrips marii Girault, 1928
Taeniothrips eriobotryae Moulton, 1928
Physothrips emersoni Girault, 1927
Thrips io Girault, 1927
Thrips partirufus Girault, 1927
Thrips pallipes Bagnall, 1926
Thrips versicolor Bagnall, 1926
Bregmatothrips theifloris Karny, 1921
Physothrips pallipes Bagnall, 1916
Physothrips albipes Bagnall, 1916
Physothrips hawaiiensis Karny, 1914
Thrips albipes Bagnall, 1914
Thrips nigriflava Schmutz, 1913
Thrips sulphurea Schmutz, 1913
Euthrips hawaiiensis Morgan, 1913
Common name
Banana flower thrips
Present taxonomic position
Family: Thripidae Stephens, 1829
Subfamily: Thripinae (Stephens) Karny, 1921
Genus: Thrips Linneaeus, 1758
Genus description
The genus Thrips L., 1758
There are nearly 300 species currently recognized in the genus Thrips making this genus one of the largest taxa within the order Thysanoptera. The genus was redefined progressively during the 1970's (see Mound et al. 1976), to include many species previously placed in Taeniothrips. The genus Thrips now includes a range of species, some with the antennae 7-segmented, others 8-segmented, and a few with the number of segments varying between 7 and 8. Similarly, some species have few setae on the fore wing first vein, whereas others have a complete row of setae on this vein. The species with a complete setal row on the first vein were placed from some taxonomists in the genera Isothrips or Isoneurothrips. However, all of the species in Thrips have the following character states: antennal segments III & IV with forked sense cone, absence of ocellar setae I, pronotum with 2 pairs of elongate posteroangular setae, paired ctenidia laterally on the tergites V-VIII, tergite VIII ctenidium arising posterior to the spiracle (in contrast to species of the genus Frankliniella). Other character states, such as number of antennal segments, number of setae on the fore wing veins, and number of discal setae on the abdominal sternites are variable between species (Mound & Masumoto 2005; Nakahara 1994; Palmer 1992). Identification keys are available for the species of this genus from many parts of the world. Of particular importance is the published key by Mound (2010) for members of the genus Thrips from Afro-tropical region as well as previous Lucid keys from Moritz et al. (2001, 2004, 2009).
Species description
Typical key character states of Thrips hawaiiensis
Coloration and body sculpture
Body color: mainly brown to dark brown or distinctively bicolored
Surface of head, pronotum and fore legs: without obvious or with weakly reticulate sculpture
Antennae
Number of antennal segments: 7-8
Antennal segment I: without any setae on dorsal apical margin
Antennal segment II: without an exceptionally long seta at the inner apex
Antennal segment II shape: symmetric
Antennal segment III shape: symmetric
Form of sense cones on antennal segments III and IV: emergent and forked on segments III and IV
Length of antennal segment III and IV: antennal segment III similar in length to segment IV
Forked sense cone on antennal segment IV: scarcely extending beyond base of segment V
Antennal segment IV and V: without a hyaline ring near the base
Antennal segment VI bears: not a remarkably dagger-shaped sensorium
Head
Distance between bases of ocellar setae III: greater than width of first ocellus
Head: not prolonged in front of compound eyes
Postocular setae II: about half as long a setae I
Ocellar setae I: absent
Length of ocellar setae II: shorter than setae III
Ocellar setae III: arising on anterior margin of, or in front of ocellar triangle
Ocelli: present
Length of postocular setae: not alternating short and long setae
Number of ocellar setae: 2
Prothorax
Number of pairs of anteromarginal minor setae: 5-6
Number of pairs of long anteroangular setae: 0
Number of pairs of long posteroangular setae: 2
Number of pairs of elongate pronotal setae: 2
Number of pairs of posteromarginal minor setae: 3-4
Pronotal blotch or internal apodeme: absent
Pronotum shape: broadly rectangular
Pronotum posteromarginal/posteroangular setae: S2 longer than S3, not equal in length
Mesothorax
Lines of sculpture around the anterior pair of campaniform sensilla on mesonotum: present
Mesosternal furca: with median spinula
Metathorax
Metanotal campaniform sensilla: present
Metanotal median setae: S1 at anterior margin
Metanotum with dominant sculptured triangle medially: absent
Metasternal furca: without spinula
Shape of metathoracic furca: transverse, V-shaped
Metanotal median setae length: longer than lateral metanotal setae
Wings
Fore and hind wings: present, more than half as long as abdomen (macropterous)
Fringe cilia arising: from sockets
Fore wing veins: present
Fore- and hind wing surface: covered with microtrichia
Apex of fore wing: with prominent terminal setae
Fore wing anterior margin (costal vein): with setae and cilia but cilia longer than setae
Fore wing clavus - number of marginal setae: 5
Fore wing clavus - terminal veinal seta: longer than subterminal seta
Fore wing costal fringe cilia: arising at anterior margin of wing
Fore wing first vein: distinct from costal vein
Fore wing first vein setal row: incomplete, with setae not closely and uniformly spaced
Fore wing second vein setal row: complete, setae uniformly spaced
Fore wing shape: mainly parallel sided or margins run continuously towards each other
Fore wing surface: not reticulate
Fore wing first vein number of setae on distal half: 3
Fringe cilia on posterior margin near apex: distinctly wavy (undulated)
Length of fore wing costal setae at middle of wing: longer than half of median wing width
Shape of fore wing apex: with mainly posterior margin curved to join anterior margin
Fore wing extreme apex color: dark
Fore wings: uniformly dark or shaded, but with base or sub-base pale
Legs
Fore tibia: not prolonged around fore tarsus
Mid and hind tarsi: with two segments
Color of fore tarsi: pale or yellow, sometimes apical shaded or brown
Abdomen
Pleurotergal discal setae: absent
Pleurotergites: not covered in microtrichia
Number of pleurotergal discal setae: 0
Number of discal setae on sternites III to VI: 10-25
Sternite II: with marginal setae and few discal setae
Sternites IV, V and VI: with marginal setae and discal setae medially
Pairs of posteromarginal setae on sternites V and VI: 3
Sternite VII median posteromarginal setae S1: arising in front of posterior margin
Sternite VII: with marginal setae and discal setae present on median area
Surface of lateral thirds of abdominal tergites: without regular rows of fine microtrichia
Number of lateral marginal setae on tergite II: 4
Sculpture of tergites II to VIII: with one or without transverse lines of sculpture between median pair of setae S1
Tergites II to VII median setal pair: no more than 0.3 as long as median length of tergite
Craspedum on tergites IV to VI: absent
Tergites IV and V median setal pair: shorter than distance between their bases
Markings on tergites IV to VI: without shaded area medially
Tergites V to VII: with ctenidia laterally
Craspedum on tergite VIII: without craspedum medially and toothlike microtrichia laterally
Tergite VIII ctenidia: posteromedial to spiracle
Tergite VIII posteromarginal comb of microtrichia: present and complete medially
Tergite VIII shape of posteromarginal microtrichia: short and irregular in length
Color of tergites IX and X: dark or brown
Tergite X: not tubular, longitudinally incomplete
Setae on abdominal tergite X: all setae slender

Similar or related species
Thrips hawaiiensis is very similar to some other Thrips species - like Thrips acaciae, Thrips brevisetosus, Thrips florum, Thrips gowdeyi and Thrips simplex. Thrips hawaiiensis has sternites III-VII with at least 1 pair of discal setae and pleurotergites without discal setae (Thrips australis, Thrips microchaetus, Thrips subnudula and Thrips tenellus, all of them have sternites III-VII with at least 1 pair of discal setae and pleurotergites with discal setae; Thrips orientalis and Thrips parvispinus with discal setae on sternites III-VI but not on sternite VII, and without discal setae on pleurotergites; Thrips nigropilosus, Thrips palmi, Thrips pusillus and Thrips tabaci, all of them have sternites and pleurotergites without discal setae).
Inside this group almost all species have the ocellar setae III on head arising on anterior margin of, or in front of, ocellar triangle, and the fore wing first vein with less than 5, mostly 3, setae on distal half (only Thrips simplex with ocellar setae III arising within ocellar triangle anterior to tangent of anterior margin of hind ocelli, and fore wing first vein with 6-10 setae on distal half). Most of them exhibit campaniform sensilla on metanotum (except for Thrips gowdeyi and Thrips simplex without metanotal campaniform sensilla), and 2 pairs of elongate pronotal posteroangular setae (except for Thrips brevisetosus without elongate pronotal setae). In Thrips hawaiiensis as well as Thrips florum metanotal median setae arise at anterior margin (compared to Thrips acaciae, Thrips brevisetosus, Thrips gowdeyi and Thrips simplex with median setae arise behind anterior margin). As in Thrips florum, the species have 7- or 8-segmented antennae (in Thrips acaciae and Thrips brevisetosus antennae 7-segmented; in Thrips gowdeyi and Thrips simplex antennae 8-segmented). Compared to Thrips acaciae and Thrips brevisetosus with mainly equiangular reticulation on metanotal median area, in other species the metanotal median area exhibit transverse sculptured lines at anterior, but longitudinal or equiangular reticulations on posterior half (Thrips gowdeyi and Thrips simplex), always longitudinal and parallel sculptured lines on posterior half (Thrips florum), or longitudinal and parallel sculptured lines or equiangular reticulations on posterior half (Thrips hawaiiensis). Furthermore, Thrips hawaiiensis has pleurotergites not covered in microtrichia (like in Thrips brevisetosus and Thrips florum; in Thrips acaciae, Thrips gowdeyi and Thrips simplex pleurotergites are covered in rows of fine microtrichia, situated on widely spaced lines of reticulation). Thrips hawaiiensis is very similar to Thrips florum, but on head the postocular setae II are about half as long as setae I, the mesonotum has lines of sculpture between and around the anterior pair of campaniform sensilla, and the terminal seta of fore wing clavus is longer than subterminal seta. Whereas Thrips florum possesses postocular setae II which are minute and much smaller than half length of setae I, a mesonotum without lines of sculpture close to anterior pair of campaniform sensilla, and in most individuals the terminal seta of fore wing clavus is shorter than subterminal seta.
Species of the genus Thrips are similar to species of Stenchaetothrips, Microcephalothrips abdominalis, Larothrips dentipes and Fulmekiola serrata because of tergites V-VIII bear a pair of ctenidia laterally, which is placed on tergite VIII posteromedial to the spiracle, and all species have no ocellar setae I on head. In contrast to species with craspedum on tergites II-VII (Microcephalothrips abdominalis, Larothrips dentipes and Fulmekiola serrata), species of Thrips and Stenchaetothrips have no posteromarginal craspedum on tergites and sternites. Species of the genus Thrips as well as Fulmekiola serrata and species of Stenchaetothrips have 2 pairs of elongate posteroangular setae (Microcephalothrips abdominalis with 2 pairs of moderately elongate pronotal setae and Larothrips dentipes without elongate setae). Compared to the species of Thrips, Microcephalothrips abdominalis, and Larothrips dentipes, which have ocellar setae II on head much shorter than or about as long as III, Fulmekiola serrata and species of Stenchaetothrips have ocellar setae II much longer than III, and sternites always without discal setae.
Biology
Life history
As with other thrips species the life cycle from egg to adult is dependent on temperature. Developmental period from egg hatch to adult emergence takes 38.9 days at 10°C (but then the adults failed to oviposit), 19.4 days at 15°C, 16 days at 20°C, 10.3 days at 25°C and 8 days at 30°C (Reynaud et al. 2008).
Host plants
Polyphagous.
Crops: apple, banana, cabbage, citrus, coffee, gladiolus, mango, passion fruit, pear, rose, tea, tobacco.
Vector capacity
None identified, but possible mechanical distribution of phytopathogenic fungi and bacteria.
Damage and symptoms
Breeding in flowers of a wide range of plants and causes direct damage by puncturing flowers and fruits, inducing spot lesions, scarring, necrosis or malformations depending on the intensity of the attack. It also feeds on pollen and host plant fertility problems can be noted (Reynaud et al. 2008). Feeding produces direct damage such as necrosis, bud malformation or reduced fruit set (Childers & Nakahara 2006).
Detection and control strategies
Floral scents such as Methyl anthranilate, Anisaldehyde and Geraniol increases the catches of Thrips hawaiiensis (Murai et al. 2000a; Imai et al. 2001). They are reported to be attractive to blue clour as compared to others (Chiu and Wu, 1993). Thrips hawaiiensis is parasitized by the eulophid parasitoid, Ceranisus menes (Murai et al. 2000b). Debudding/deflowering of the male flowers after fruit set and covering fruit bunches with polythene sheets in banana can protect the crop from Thrips hawaiiensis, Thrips florum and Chaetanaphothrips.
Additional notes
-

Biogeography
Widespread in the Oriental and Pacific Region, also Jamaica, Mexico, North America, Africa. Angola, Mozambique, Nigeria,
Sierra-Leone,
Uganda.
African countries where Thrips hawaiiensis has been reported
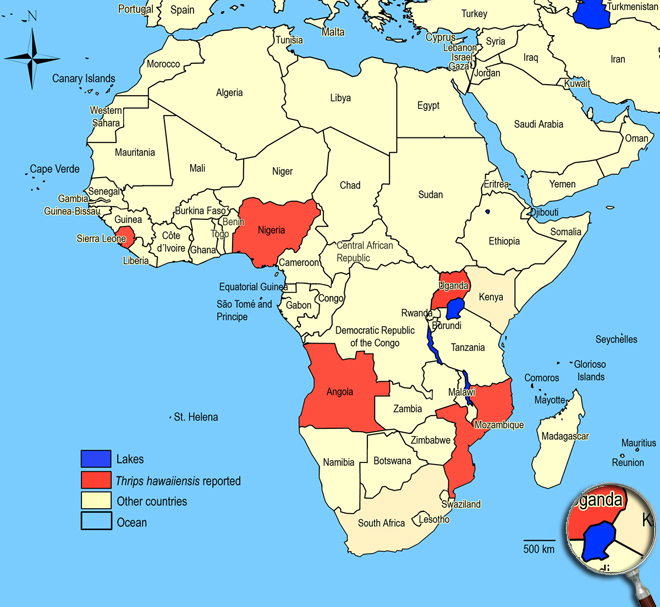
The species Thrips hawaiiensis was not observed in surveys undertaken in East Africa on vegetables and associated weeds and crops.
Please click here for survey sites of all observed thrips species of Kenya, Tanzania and Uganda.
Bibliography
Abe M & Ikegami T (2005). Susceptibility of five species of thrips to different strains of the entomopathogenic fungus, Beauveria bassiana. Applied Entomology and Zoology. 40 (4): 667-674
Anonymous (1983). CIE (Commonwealth Institute of Entomology) Thrips hawaiiensis (Morg). Distribution maps of pests, Series A (Agricultural), No. 431. London, UK, Commonwealth Agricultural Bureaux, 2 pp. http://www.cabi.org/dmpp/
Bagnall RS (1914). Brief descriptions of new Thysanoptera - II. Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 8) 13: 22-31
Bagnall RS (1916). Brief descriptions of new Thysanoptera - VIII. Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 8) 17: 397-412
Bagnall RS (1926). Brief descriptions of new Thysanoptera - XV. Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 9) 18: 98-114
Bhatti JS (1999). New characters for identification of the pest species Thrips hawaiiensis and florum (Terebrantia: Thripidae). Thrips. 1: 31-53
Chen JS & Lo PKC (1987). Differential preference of the flower-dwelling thrips, Thrips hawaiiensis (Morgan) (Thysanoptera: Thripidae), to some gladiolus cultivars. Journal of Agricultural Research of China. 36 (3): 317-326
Chen L-S (1979). Thrips infesting tea in Taiwan. Plant Protection Bulletin, Taiwan. 21: 377-382
Childers CC & Nakahara S (2006). Thysanoptera (thrips) within citrus orchards in Florida: Species distribution, relative and seasonal abundance within trees, and species on vines and ground cover plants. Journal of Insect Science. 6 (45): 1-19
Chiu HT & Wu MY (1993). Attractiveness of color trap to Thrips hawaiiensis (Morgan) (Thysanoptera: Thripidae) in the field. Chinese Journal of Entomology 13: 229 - 234
Chiu HT, Shen SM & Wu MY (1991). Occurrence and damage of thrips in citrus orchards in southern Taiwan. Chinese Journal of Entomology. 11: 310-316
Girault AA (1927). A discourse on wild animals. Published privately, Brisbane, 2 pp
Girault AA (1927). Some new wild animals from Queensland. Published privately, Brisbane, 3 pp
Girault AA (1928). A prodigeous discourse on wild animals. Published privately, Brisbane, 3 pp
Girault AA (1928). Notice of a curious professor and of native wasps and woodlice. Published privately, Brisbane, 4 pp
Girault AA (1930). New pests from Australia, VIII. Published privately, Brisbane, 5 pp
Imai T, Maekawa M & Murai T (2001). Attractiveness of methyl anthranilate and its related compounds to the flower thrips, Thrips hawaiiensis (Morgan), T. coloratus Schmutz, T. flavus Schrank and Megalurothrips distalis (Karny) (Thysanoptera: Thripidae). Applied Entomology and Zoology. 36 (4): 475-478
Jhala RC, Borad PK & Bharpoda TM (2004). Incidence of Thrips hawaiiensis (Morgan) on banana in Gujarat. Insect Environment. 10: 55
Karny H (1921). Beiträge zur Malayischen Thysanopterenfauna, IV. Thysanopteren von Hevea, V. Thysanopteren an Tee. Treubia. 2 (1): 37-83
Karny H, van Leeuwen-Reijnvaan W & van Leeuwen-Reijnvaan J (1914). Beiträge zur Kenntnis der Gallen von Java. Zweite Mitteilung über die javanischen Thysanopterocecidien und deren Bewohner. Zeitschrift für Wissenschaftliche Insektenbiologie. 10: 355-369
Ketavan C (1978). Thrips of economic importance and their control in Thailand. Report, Kasetsart University, Thailand, p. 90
Kurozawa M, Takaoka I & Naitô T (1964). Thrips infesting the tobacco plants in Japan (Thysanoptera). Japanese Journal of Entomology (Konchû). 32 (3): 402
Lee HS & Wen HC (1982). Seasonal occurrence of and injury caused by the thrips and their control on mangoes. Plant Protection Bulletin, Taichung. 24: 179-187
Lewis T (1973). Thrips: their biology, ecology and economic importance. Academic Press Inc., London Ltd., 349 pp.
Lewis T (1997). Thrips as crop pests. CAB International, Wallingford, 740 pp
Morgan AC (1913). New genera and species of Thysanoptera with notes on distribution and food plants. Proceedings of the United States National Museum. 46: 1-55 (in bibliographical references always denoted by 1914)
Moritz G (2006). Thripse. Pflanzensaftsaugende Insekten, Bd. 1, (1. Auflage). Westarp, Hohenwarsleben, 384 pp. ISBN-13: 978 3 89432 891 7
Moritz G, Morris DC & Mound LA (2001). ThripsID - Pest thrips of the world. ACIAR and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1 86320 296 X
Moritz G, Mound LA, Morris DC & Goldarazena A (2004). Pest thrips of the world - an identification and information system using molecular and microscopical methods. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN 1 86499 781 8
Moritz G, O'Donnell C & Parrella M (2009). Pest thrips of North America. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN-13: 978 1 86499 940 2
Moulton D (1928). Thysanoptera of Japan: New species, notes, and a list of all known Japanese species. Annotationes Zoologicae Japonenses. 11 (4): 287-337
Mound LA (2010). Species of the genus Thrips (Thysanoptera, Thripidae) from the Afro-tropical Region. Zootaxa. 2423: 1-24
Mound LA & Kibby G (1998). Thysanoptera: An identification guide, (2nd edition). CAB International, Wallingford and New York, 70 pp
Mound LA & Marullo R (1996). The thrips of Central and South America: An introduction (Insecta: Thysanoptera). Memoirs on Entomology, International, Vol. 6. Associated Publishers, Gainsville, 487 pp
Mound LA & Masumoto M (2005). The genus Thrips (Thysanoptera, Thripidae) in Australia, New Caledonia and New Zealand. Zootaxa. 1020: 1-64
Mound LA, Morison GD, Pitkin BR & Palmer JM (1976). Thysanoptera. Handbooks for the identification of British insects, Vol. 1, Part 11. Royal Entomological Society of London, London, 79 pp
Murai T (2001). Development and reproductive capacity of Thrips hawaiiensis (Thysanoptera: Thripidae) and its potential as a major pest. Bulletin of Entomological Research. 91 (3): 193-198
Murai T, Imai T & Maekawa M (2000a). Methyl anthranilate as an attractant for two thrips species and the thrips parasitoid Ceranisus menes. Journal of Chemical Ecology. 26 (11): 2557-2565
Murai T, Kawai S, Chongratanameteekul W & Nakasuji F (2000b). Damage to tomato by Ceratothripoides claratris (Shumsher) (Thysanoptera: Thripidae) in central Thailand and a note on its parasitoid, Goetheana shakespearei Girault (Hymenoptera: Eulophidae). Applied Entomology and Zoology. 35 (4): 505-507
Nakahara S (1985). Review of Thrips hawaiiensis and revalidation of T. florum (Thysanoptera: Thrpidae). Proceedings of the Entomological Society of Washington. 87 (4): 864-870
Nakahara S (1994). The genus Thrips Linnaeus (Thysanoptera: Thripidae) of the New World. Technical Bulletin, USDA, Agricultural Research Service. 1822: 1-183
Palmer JM (1992). Thrips (Thysanoptera) from Pakistan to the Pacific: a review. Bulletin of the British Museum (Natural History), Entomology. 61 (1): 1-76
Palmer JM, Mound LA & du Heaume GJ (1989). 2. Thysanoptera, 73 pp. In Betts CR [ed.], CIE Guides to insects of importance to man. CAB International, Wallingford, Oxon, UK
Palmer JM & Wetton MN (1987). A morphometric analysis of the Thrips hawaiiensis (Morgan) species-group (Thysanoptera, Thripidae). Bulletin of Entomological Research. 77: 397-406
Priesner H (1934). Indomalayische Thysanopteren (VI). Natuurkundig Tijdschrift voor Nederlandsch-Indie. 94 (3): 254-290
Priesner H (1938). Materialen zu einer Revision der Taeniothrips-Arten (Thysanoptera) des indomalayischen Faunengebietes. Treubia. 16: 469-526
Reynaud P, Balmès V & Pizzol J (2008). Thrips hawaiiensis (Morgan, 1913) (Thysanoptera: Thripidae), an Asian pest thrips now established in Europe. Bulletin OEPP/EPPO Bulletin. 38: 155-160
Schmutz K (1913). Zur Kentniss der Thysanopterenfauna von Ceylon. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse, Abteilung I. Biologie, Mineralogie, Erdkunde. 122 (7): 991-1089
Tsai YP, Hwang MT & Chen HP (1992). Occurrence and damage of Thrips hawaiiensis in banana orchards. Chinese Journal of Entomology. 12: 231-237
Wang C-L (1982). Insect pests and their injury on rose. Journal of Agricultural Research of China. 31 (1): 97-101
zur Strassen R (2003). Die terebranten Thysanopteren Europas und des Mittelmeer-Gebietes. Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und nach ihrer Lebensweise, 74. Teil. Goecke & Evers, Keltern, Germany, 277 pp
----
Web links
Mound´s Thysanoptera pages
Thysanoptera Checklist
ICIPE Thrips survey sites
UNI Halle & Thrips sites
Thrips of California












